|
|
|
DEVELOPMENTAL BIOLOGY 3235 |
|
|
|
|
|
|
|
|
|
| Lab 1 |
|
|
Most of the material presented here is taken from the online C. elegans Community resourses. See WormBase, C. elegans WWW server, WormAtlas, and C. elegans II at NCBI. |
|
|
|
|
 |
|
Why C. elegans? Sea urchins have told us much about embryogenesis. They are suited well for study in the lab; however, they do not tell us much about the genetics involved in embryogenesis. As we have seen in the last few lectures, drosophila, with its short generation time (14 days) and its ability to be kept generation after generation under lab conditions, continues to be a useful tool to study the genetics of embryology. However, some aspects of fly development are complex and difficult to study.
|
|
|
|
 |
|
|
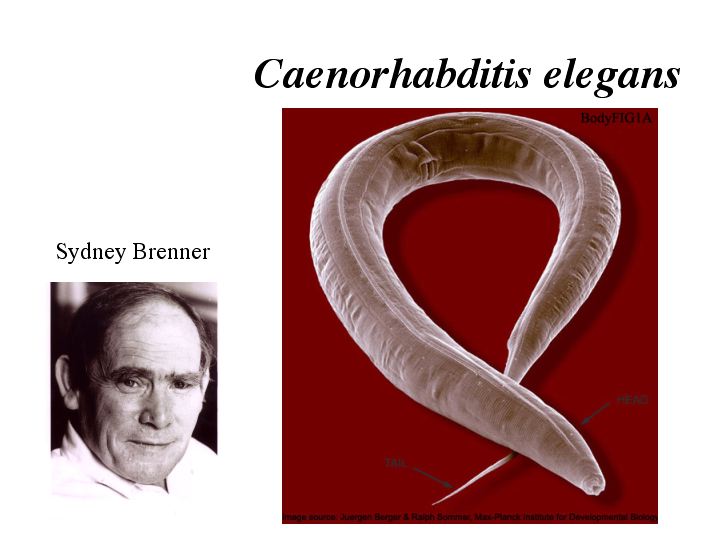
| In 2002, Sydney Brenner was 1 of the 3 recipients of the Nobel Prize in Medicine. He was awarded this honor for establishing the nematode Caenorhabditis elegans as a model system to study the genetics of development. C. elegans is a free living round worm found in the soil. It is easy to maintain generation after generation under lab conditions. It can be grown on petri plates seeded with bacteria or in liquid culture. These worms can be frozen for storage and will recover soon after thawing. Their generation time is only three days and since the majority of worms are hermaphrodites they can self-fertilize so you don’t have to set up a cross each generation. Infrequent chromosome nondisjunction gives rise to male worms so you also have the option of cross-fertilization. |
|
 |
|
| The worms are transparent allowing you to see the internal features of the worm. The worm consists of a simple digestive tract consisting of the pharynx, intestine and anus. Each worm can produce a brood size up to 300 without cross-fertilization. Oocytes migrate from the distal arms of the gonad and around to the spermatheca where sperm is stored. The oocyte enters the spermatheca where it is fertilized, and then passes into the uterus. (Worm Atlas) |
|
|
 |
|
| Each worm consists of exactly 959 somatic cells. John Sulston, the second of the 3 recipients of the Nobel Prize in Medicine in 2002 was able to identify the cell lineage of all 959 cells. The diagram above shows this lineage. Each vertical line above represents a single cell. Each horizontal line represents a cell division. Any single cell will follow the same pattern of cell divisions in the worm, so the entire anatomy of the worm is known on a cellular level. Because of this, circuit diagrams of the entire nervous system of the worm have been created. |
|
|
 |
|
|
|
| In addition to the two Nobel recipients mentioned, the third recipient of the Nobel Prize in Medicine last year went to Bob Horvitz. Bob Horvitz noted that there were actually 1090 somatic cells in the adult worm, but 131 of these cells underwent programmed cell death. He then performed screens to identify mutants where some of these cells that were programmed to die actually lived; and, in contrast where specific cells that normally lived underwent apoptosis. Up until the late 1980’s apoptosis was a theory not readily accepted by all scientists. Bob Horvitz has now identified many of the genes involved in this process (Horvitz Lab Web Site) |
|
|
 |
|
|
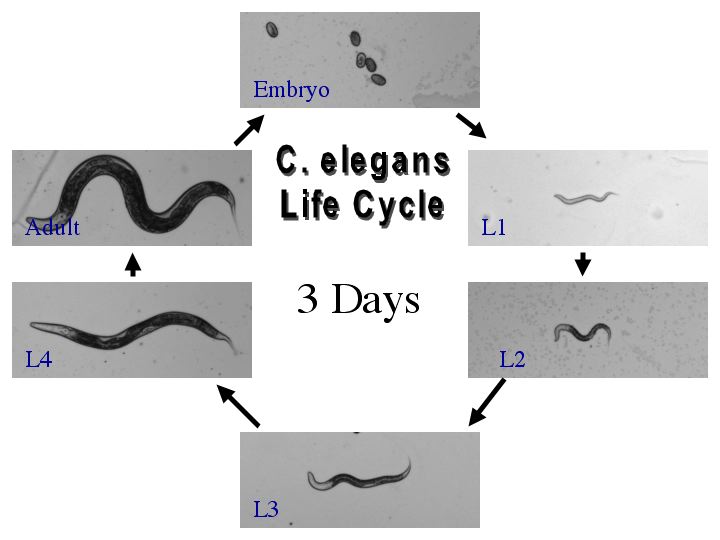
| The generation time is only three days. After hatching, the worm will go through four larval stages before becoming an adult. As an adult the worm will live up to two weeks. |
|
 |
|
|
|
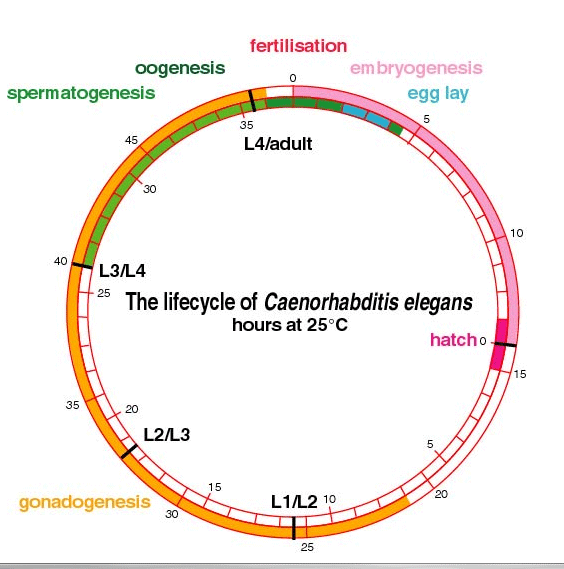
| This timeline shows the lifecycle of C. elegans. The outer circle show times since fertilization and the inner circle shows times from hatch. This figure and addition introductory lessons on C. elegans biology can be found at Mark Blaxter's, The Introduction to C.elegans. |
|
 |
|
|
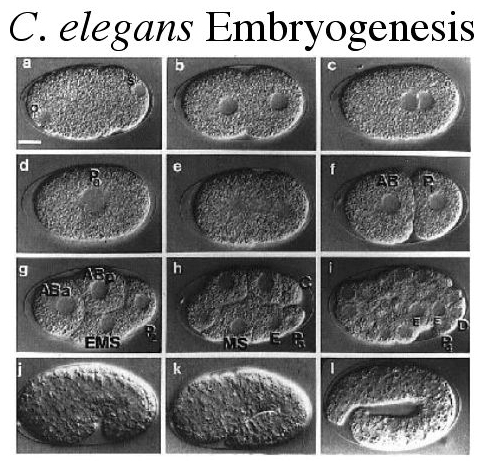
| Embryogenesis is easy to observe in living worms since they are transparent. The entire process of embryogenesis takes only 16 hours from the time of fertilization until the time of hatching. In this figure we can see the pronuclei of the oocyte and sperm. The oocyte pronucleus migrates to the posterior to unite with the sperm pronucleus. (Worm embryogenesis movies) |
|
 |
|
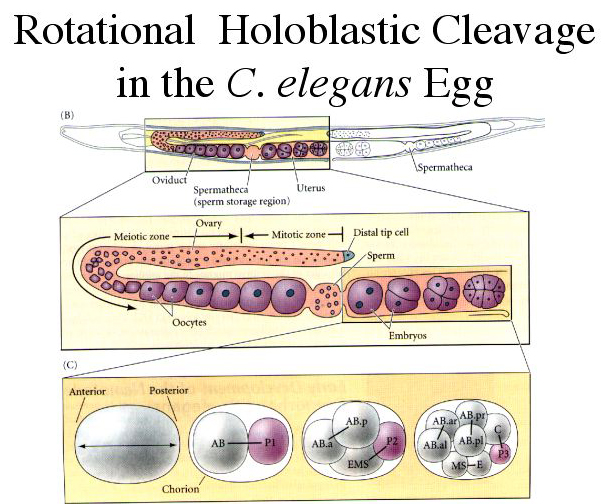
| C. Elegans embryos undergo rotational holoblastic cleavage. The initial 4 or 5 cleavages take place within the uterus of the hermaphrodite and the remaining cleavage events take place outside of the mom. |
|
 |
|
|
| On the left is an illustration of the initial cleavage events, and on the right is the cell lineage. In the first four cell divisions, founder cells (AB, MS, C, E, D, & P4) are established that will give rise to specific cell fates. The next step was to discover the genes that are involved in the establishment of these cell fates. We can see that some cell fates are established just after the first asymmetric division. This shows that the A-P polarity was established in the Po cell. Next we’ll look at how this occurs. |
|
 |
|
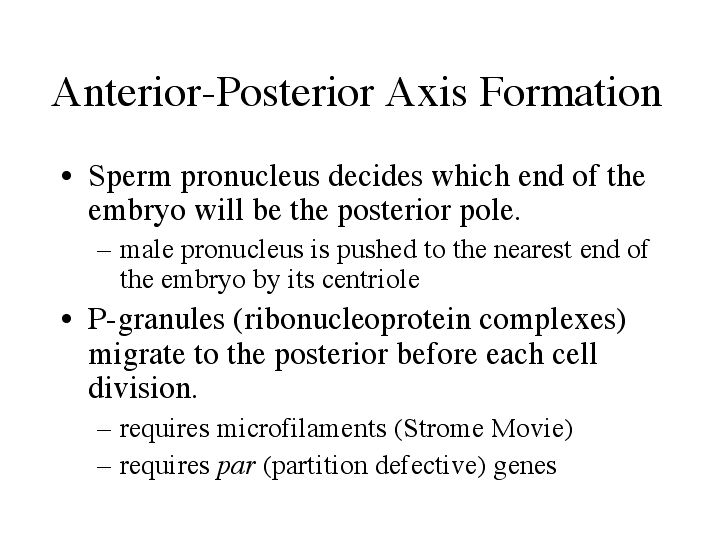
| Unlike drosophila, polarity in C. elegans isn’t decided until after sperm entry. Until then, either end of the embryo can become the posterior. The closest side that the sperm enters will become the posterior of the embryo. The sperm centriole is thought to enucleate microtubules which push the sperm pronucleus to the side nearest the point of entry, making the sperm the initial cue of axis formation. Another indication of A-P polarity came when ribonucleoprotein complexes (P-granules) were observed migrating to the posterior pole before each cell division. P-granules are thought to contain proteins that specify germ cell fate. This movement required actin microfilaments. (Strome Movie). The movement of P-granules also depends on a set of maternal genes called par for partition defective. |
|
 |
|
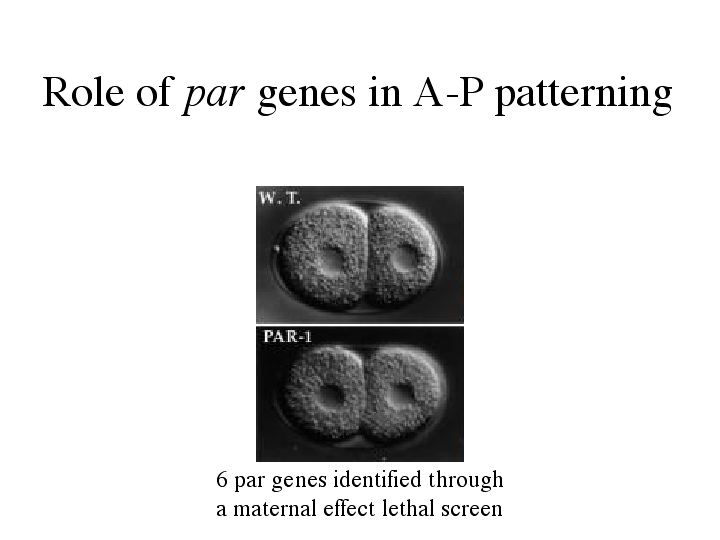
| Role of Par genes. 6 par genes have been identified through a maternal effect lethal screen. Mutations in 5 of the 6 par genes cause the first cell division to be symmetric instead of asymmetric. |
|
|
|
|
 |
|
|
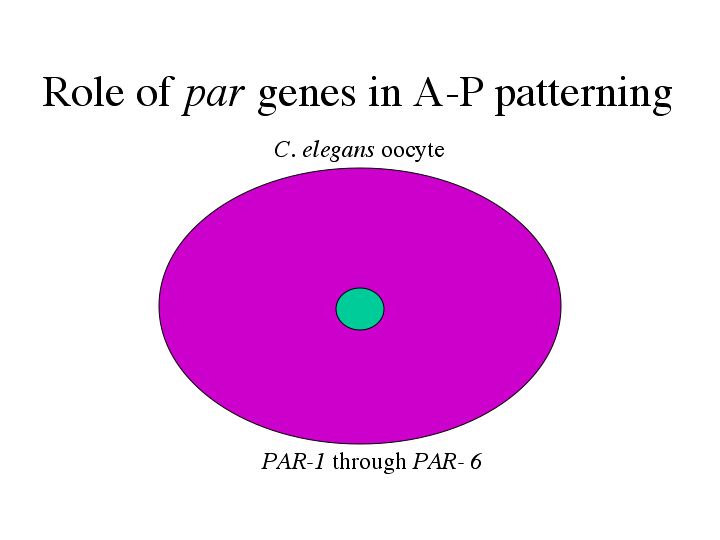
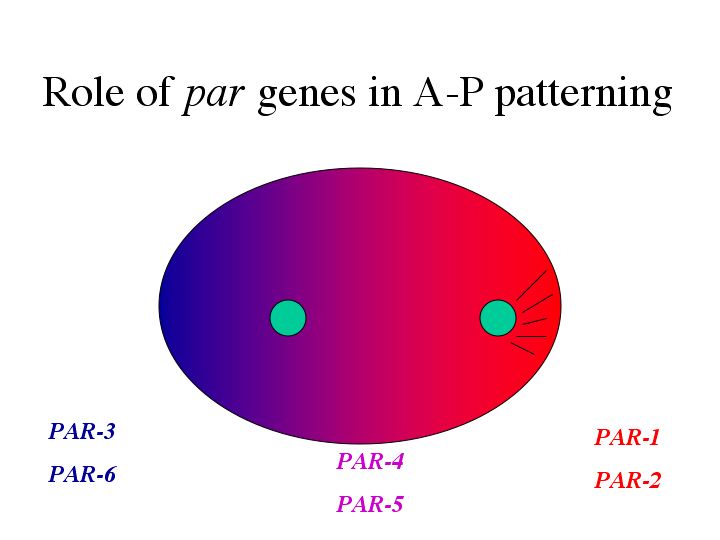
| Antibody staining showed that all 6 PAR proteins are located throughout the oocyte before fertilization. |
|
|
|
 |
|
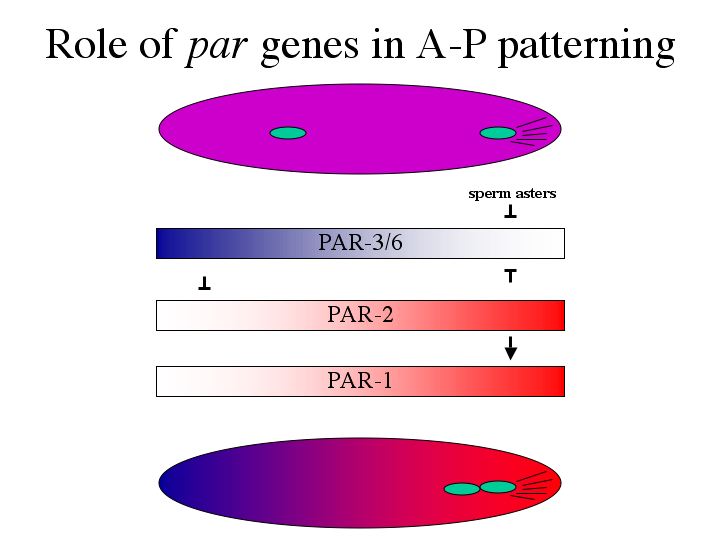
| However, after fertilization PAR-3/6 are localized to the anterior, PAR-1/2 to the posterior, while PAR-4/5 remain throughout the embryo. |
|
|
|
 |
|
| An unknown signal from the sperm asters destabilizes PAR-3/6 in the posterior to establish the initial gradient. At the anterior, PAR-3/6 destabilize PAR-2 which enhances the gradient by destabilizing PAR-3/6. PAR-2 then stabilizes PAR-1. By the time pronuclei fuse the PAR-3/6, PAR-1/2 gradient has been established. It doesn’t stop there, though. |
|
|
 |
|
| PAR-1 then destabilizes MEX-5/6. MEX-5/6 destabilize germline proteins such as PIE-1 and other components of the P-granules. Although P-granules are destabilized in the anterior, we have seen P-granules actually moving from the anterior to the posterior. This cytoplasmic movement of P-granules is not fully understood. MEX-5/6 and the germline proteins are now also in an A-P gradient by the time the cell divides. |
|
 |
|
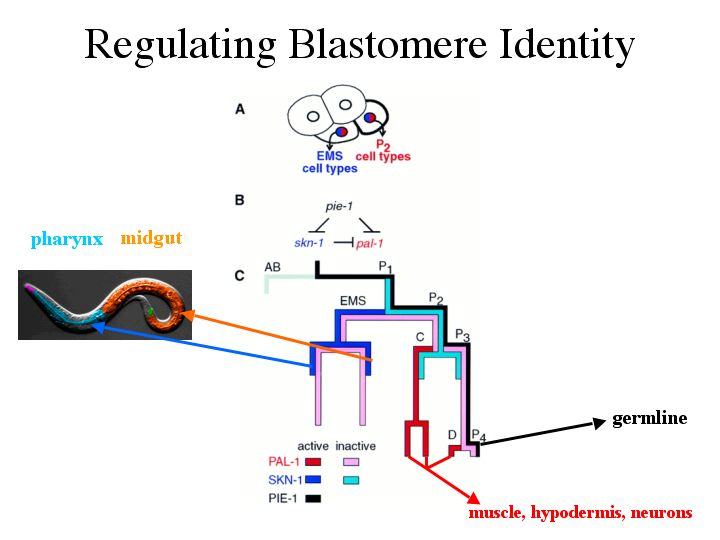
| We can now begin to understand the establishment of components needed to set up transcription factors that regulate blastomere identity. Two of these transcription factors, skn-1 and pal-1, are required to develop the gut of the worm and muscle/hypodermis/neurons of the posterior, respectively. I’ve shown how PIE-1 is established in the P1 cell after the first cell division. PIE-1 is necessary for germline fate. SKN-1 and PAL-1 are also established in the P1 cell by a yet unknown mechanism. It has been proposed that pie-1 represses skn-1 and pal-1 activity in the P1 cell, and thus repressing blastomere identity in this cell. After the P1 cell divides, PIE-1 remains active in only the posterior P2 cell so skn-1 is no longer repressed in the EMS cell. The EMS founder cell is then fated to give rise the the pharynx and midgut. Pal-1 continues to be repressed in the EMS cell by skn-1 and in the P2 cell by pie-1 until the third cell division. Pal-1 is then active in the C and D founders to promote development of the muscle, hypodermis, and neurons in the posterior. |
|
| The genetics behind A-P patterning sounds entirely different to that what you have learned in fly. First, think of how different cleavage events are in an insect to that of a worm. A-P patterning is determined in the fly oocyte while patterning doesn’t occur in the fly until after fertilization. However, the par homologs looked at in drosophila seem to be involved in the initial patterning events of the oocyte. Other genes involved in patterning are apparent, such as pal-1 discussed in the previous slide. The drosophila homolog is caudal, also expressed the in posterior. PAR science paper |
|
|
|
 |
|
 |
|
|
| What can worms teach us about ourselves? Now I’m going to switch gears and discuss a topic that shows how we can use worms to discover genes involved the process of organ development. This is work done in Susan Mango’s lab at the Huntsman Cancer Institute. |
|
 |
|
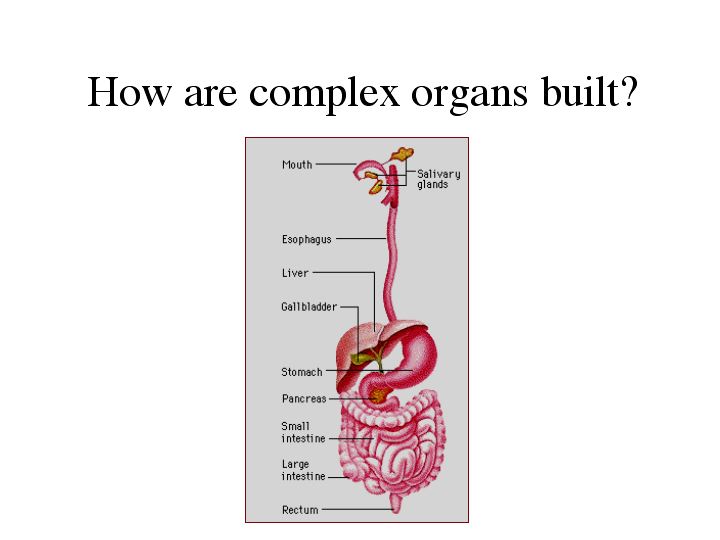
| How are complex organs built? Think of all that organ develop entails and the hallmark of organ development. Millions of different cells come together to perform a specific function. These cells consist of many different cell types. How is the function of all these cells coordinated? How are all of these cells given identity to become a specific organ? What genes are regulating this process and how are they doing it? Since organ development in mammals consists of a complex multistep process, the answer to these questions are difficult to obtain. |
|
 |
|
|
|
| So instead, we use the C. elegans pharynx as a model of organ development. The pharynx is used to grind and pump food into the intestine. |
|
 |
|
| Like other organs, the C. elegans pharynx also contains different cell types. Epithelial cells, neurons, muscle cells, gland cells, and valve cells all come together to create the pharynx. |
|
 |
|
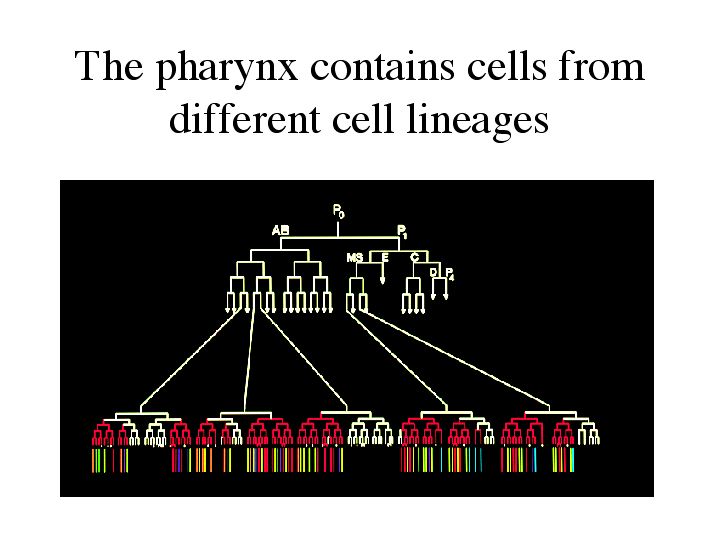
| In addition, the pharynx contains cells from different cell lineages. The model of organ development would be simplified if an organ was created from one cell that continues to divide until the whole organ is formed. We can see that this is not the case with the pharynx. The cells in red indicate cells that will give rise to the pharynx. The different colors underneath indicate the different cell types. How can this be achieved? |
|
 |
|
|
|
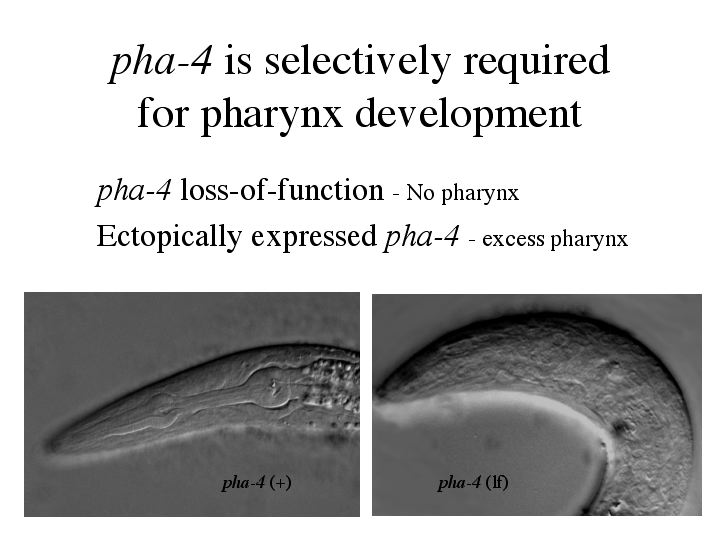
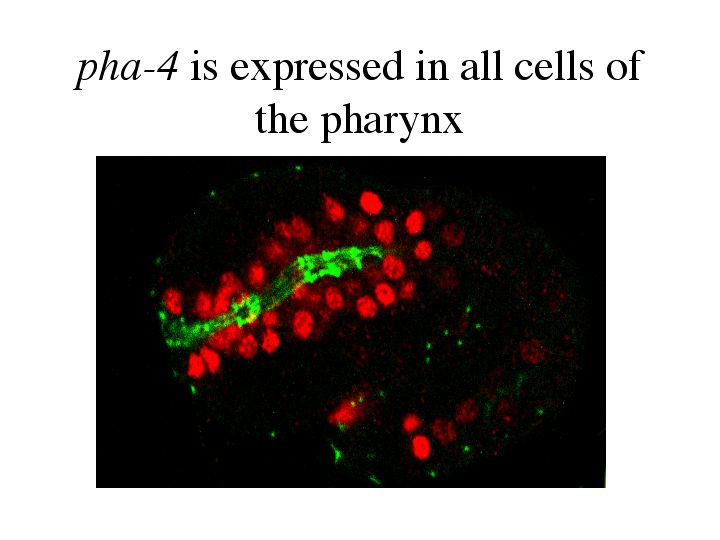
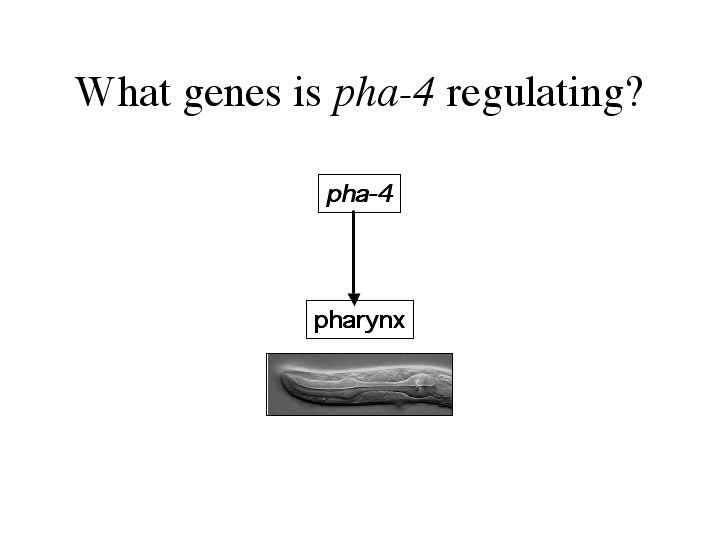
The answer to this comes from the transcription factor, pha-4. pha-4 is selectively required for pharynx development. The pha-4 loss of function phenotype is a worm that lacks a pharynx. These worms complete embryogenesis, but starve to death after hatching because they are unable to eat. Ectopic expression of pha-4 will create ectopic pharyngeal precursors, showing that pha-4 is a master regulatory gene controlling pharyngeal development. |
|
 |
|
| pha-4 is expressed in every cell that will become part of the pharynx. This is an embryo at the two fold stage. The antibody against pha-4 is shown in red. |
|
 |
|
|
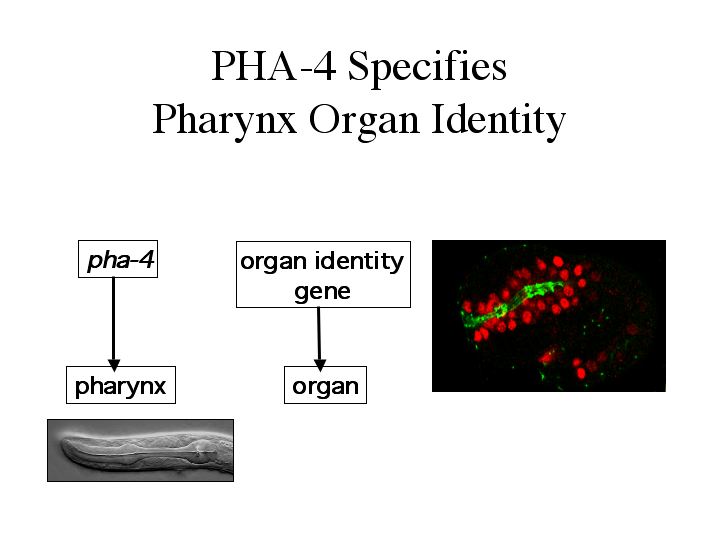
| pha-4 can be thought of as an organ identity gene. pha-4 gives rise to the pharynx. Other organs may require other organ identity genes. This is the case in eye development of both the fly and vertebrates. The loss of the pax-6/eyeless gene will cause the loss of eyes in both the fly and vertebrates. Ectopic expression of eyeless in the fly will induce ectopic eyes. |
|
 |
|
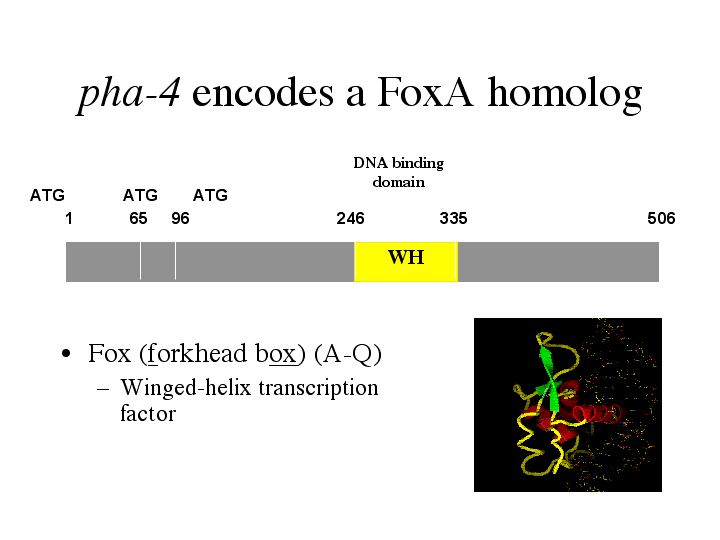
| pha-4 encodes the FoxA homolog. Fox proteins, which stands for forkhead box - the fly homolog, fall into several subclasses labeled A-Q. Each has a winged-helix DNA binding domain. |
|
|
 |
|
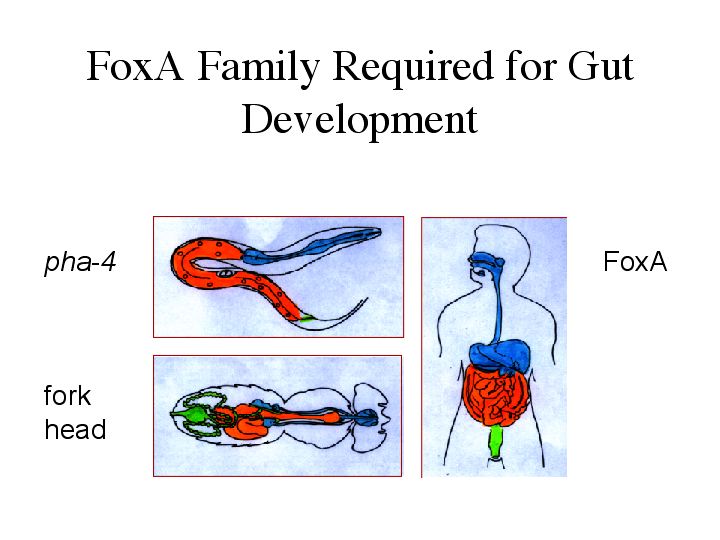
| pha-4 is a member of the FoxA subclass. FoxA family members have been found in all metazoans looked at so far, and in each case studied the proteins are required for gut development. Shown here are the similarities between the guts of the worm, fly, and human. The forgut is in blue, the midgut in orange, and the hindgut in green. |
|
|
|
 |
|
| How is pha-4 conferring organ identity. What genes are regulated by pha-4? |
|
 |
|
|
|
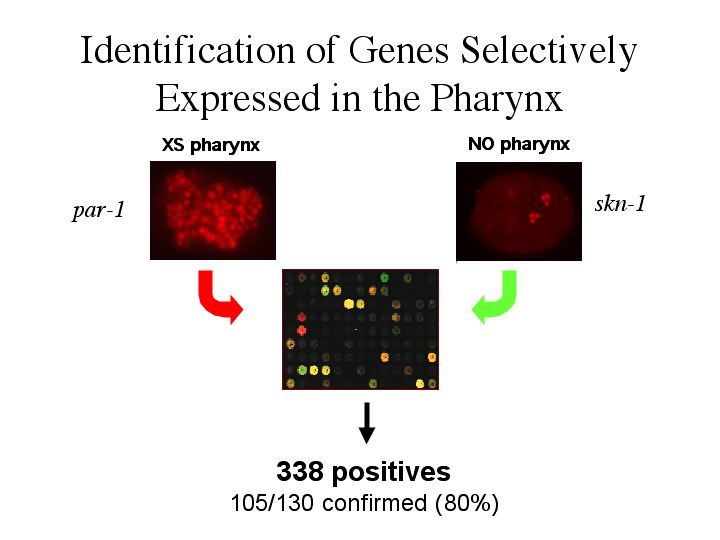
| To answer these questions the expression profile of embryos with excess pharynx were compared to embryos with no pharynx. An anti-pha-4 stain is shown in red. Notice that par-1 embryos give more pha-4 expressing cells while skn-1 gives little. Why is this. Remember that par-1 embryos do not have a posterior cell fate, which gives rise to more pharynx forming cells as a consequence. skn-1 was the transcription factor required for gut development. When skn-1 is absent, the cells that would have given rise the the pharynx and midgut are transformed into hypodermal cells. RNA was prepared from both of these samples. Fluorescently labeled cDNA was made from the RNA (red label for the par-1 embryo cDNA and green for the skn-1 embryo cDNA). These cDNA’s were hybridized to a gene chip containing oligos for all 19,000 genes. If a gene is expressed higher in the par-1 embryos where there are more pha-4 expressing cells than in the skn-1 embryos, you will see a red dot. Conversely, if a gene is down regulated in the par-1 embryos you will see a green spot. A yellow spot would indicate no change in expression between the par-1 and skn-1 embryos. This microarray experiment showed that 338 genes were upregulated in embryos with more pha-4 expression (par-1) than in embryos without pha-4 expression (skn-1). Expression data was available for 130 of these genes. As a conformation that this experiment worked, 105/130 showed expression in the pharynx. |
|
|
 |
|
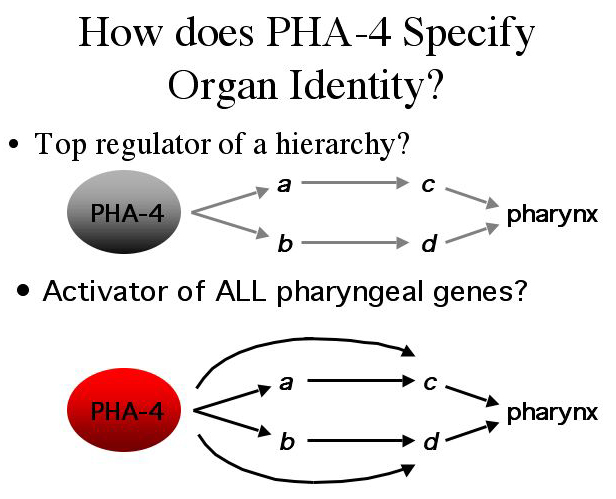
| How does pha-4 regulate all of these genes to give rise to the pharynx? Could it be that it regulates just a few pharyngeal genes making it the top regulator of a hierarchy? |
|
| Or could it be acting on all pharyngeal genes? |
|
|
 |
|
|
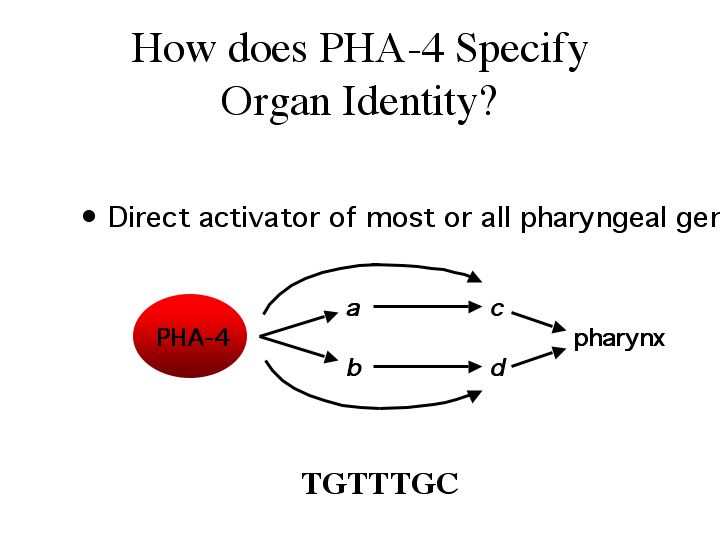
| The pha-4 binding site (TGTTTGC) was enriched in promoter regions of most of the 338 positives, suggesting that pha-4 is a direct activator of most or all pharyngeal genes. Experiments mutating the pha-4 binding sites in several of these genes reduced expression in the pharynx, proving that the pha-4 binding sites are real. Coordination of organ development may occur through an organ identity gene, like pha-4, to which directly activates most or all organ genes at the transcriptional level. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|































































